The Learning Center
Contamination Problem
Foreign body contamination can result in benign changes, severe complications, and in some cases death. 3,4,6,16
Starch contamination has been snuffed out. Now it’s time to lose the lint.
Cellulose fibers, lint, released from commonly used sterile cotton products like cotton surgical towels, gauze, non-adherent TELFA™ pads, RAY-TEC® sponges, and lap pads are documented sources of foreign body contamination.
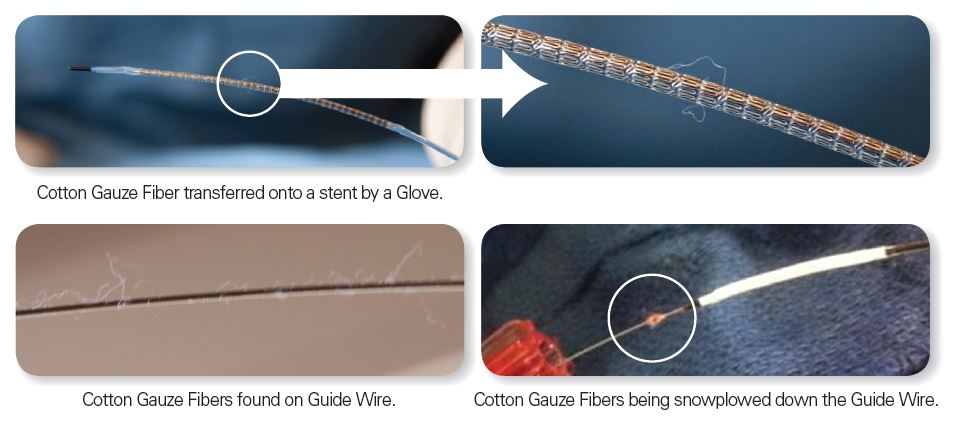
Lint from cotton products can be introduced into patients four ways:
- Contact Transfer: Cotton products used in the procedure come into contact with the medical device which includes transfer from the handler’s gloves and draped standard cotton surgical towels.
- Fluid Transfer: When cotton products (i.e. gauze, TELFA, etc.) are dunked in bowls and fluid to wipe instruments, and the same fluid is drawn up and used for sheath, catheter flushing, or as irrigation in a wound/incision.
- Airborne Transfer: When standard cotton products shed particulates into the air that land on a device or in a wound/incision.
- Direct Transfer: When cotton products (i.e. RAY-TEC sponges, standard lap pads, etc.) are placed directly in the wound or incision.
Linting cotton products used in interventional procedures have been documented to cause the following complications, and others:
- occlusive granulomas
- infection
- necrosis
- arteritis or aterial occlusion
- infarct
- stroke
- phlebitis
- kidney failure
- seizures
- misdiagnosed carcinomas
- death
Complications more commonly associated with linting cotton products used in open body cavity surgery:
- granulomas
- adhesions
- infection
- death
The number of bacteria needed to initiate an infection drops from ~100,000 to ~100 when foreign body debris is present.18
Presentations about fiber and lint transmission and contamination
Syntervention offers products designed to eliminate preventable lint and fiber contamination.
Many medical devices, particularly implants and intravascular devices, are manufactured to strict requirements concerning cleanliness, particulate, and fiber content. However, once the sterile medical devices are opened for a procedure, they become exposed to a number of foreign bodies and contaminating elements, primarily cotton based products previously described.
Essentially the cleanliness, particulate, and fiber requirements the device was manufactured to no longer benefits the patient when the device is subjected to the elements in the operating room or the interventional lab. Therefore, it is imperative, that the operator and support staff keep the sterile medical devices and instruments as clean as possible prior to every insertion into a patient’s body.
SWASHER Ultra Low-Lint Surgical Towel
SWIPER Medical Device Foam Wiper
GUIDE-KLIP Medical Device Retaining Tool
Reference Articles
Video Library
Syntervention in the Press
Purchasing
888.505.8802 main
888.853.6559 fax
info@syntervention.com